6 Proven Strategies for Transitioning from Forensic Biology to Clinical Trials
Imagine the thrill of solving mysteries in forensic biology, now applied to groundbreaking clinical trials. The transition from forensic biology to clinical trials can open up exciting opportunities in clinical research careers and pharmaceutical industry jobs.
As a life coach, I’ve helped many professionals navigate these challenges. In my experience, transitioning careers can seem daunting but is entirely achievable with the right strategy. Developing clinical trial management skills and exploring CRA (Clinical Research Associate) positions can be excellent starting points.
In this article, you’ll learn proven strategies to transition from forensic biology to clinical trials. We’ll cover tips on identifying transferable skills, networking, and gaining relevant certifications like GCP (Good Clinical Practice). You’ll also discover insights into clinical data management and regulatory affairs in clinical trials.
Let’s dive in.

Understanding the Challenges of Career Transition
Switching from forensic biology to clinical trials is no small feat. Many clients initially struggle with the steep learning curve when transitioning to clinical research careers.
You have to adapt to new terminologies, methodologies, and industry standards, including Good Clinical Practice (GCP) certification and clinical trial protocol development.
This career shift from forensic biology to clinical trials can feel overwhelming. It impacts your long-term career trajectory and professional growth in pharmaceutical industry jobs.
The uncertainty can be stressful.
You might need to acquire entirely new skills and knowledge specific to clinical trials, such as patient recruitment strategies and clinical data management. This requires time, effort, and dedication.
But, it’s entirely possible with a strategic plan, especially when aiming for CRA (Clinical Research Associate) positions.
In my experience, the most successful transitions from forensic biology to clinical trials start with recognizing these challenges. Acknowledge them, and you’ll be better prepared to tackle them head-on, whether it’s mastering regulatory affairs in clinical trials or site selection and feasibility in clinical studies.

Strategic Steps for Transitioning to a Clinical Trials Career
Overcoming this challenge requires a few key steps. Here are the main areas to focus on to make progress in transitioning from forensic biology to clinical trials:
- Identify transferable skills from forensic biology: List and match your skills to those needed in clinical research careers and pharmaceutical industry jobs.
- Research clinical trials industry and roles: Conduct thorough research to understand the landscape, key roles, and clinical trial management skills required.
- Network with clinical research professionals: Attend conferences, join forums, and conduct informational interviews to learn about CRA (Clinical Research Associate) positions.
- Gain relevant certifications (e.g., ACRP, SOCRA): Research, enroll in courses, and prepare for certification exams, including GCP (Good Clinical Practice) certification.
- Seek internships or entry-level positions: Apply for internships and entry-level positions for hands-on experience in clinical data management and regulatory affairs in clinical trials.
- Tailor resume to highlight applicable experience: Emphasize transferable skills and relevant experience on your resume, including any knowledge of patient recruitment strategies or clinical trial protocol development.
Let’s dive in!
1: Identify transferable skills from forensic biology
Identifying transferable skills from forensic biology is crucial for a successful transition to clinical trials. Moving from forensic biology to clinical trials requires recognizing valuable competencies.
Actionable Steps:
- List your core skills and experiences: Create a comprehensive list of your skills and experiences in forensic biology, including those relevant to clinical research careers.
- Match skills to clinical trials roles: Identify which skills align with clinical trials requirements, such as data analysis and investigative techniques applicable to CRA positions.
- Highlight analytical and investigative skills: Emphasize how your forensic biology skills can be valuable in clinical research settings and pharmaceutical industry jobs.
Key transferable skills from forensic biology include:
- Attention to detail and precision, essential for clinical trial protocol development
- Data analysis and interpretation, crucial for clinical data management
- Adherence to strict protocols, aligning with GCP certification requirements
Explanation: Recognizing your transferable skills helps bridge the gap between forensic biology and clinical trials.
This approach not only boosts your confidence but also makes you more attractive to potential employers in the clinical research field.
For example, strong analytical skills are highly valued in clinical research, where precision and attention to detail are crucial for tasks like site selection and feasibility in clinical studies.
This will help you stand out in a competitive job market for clinical trial management skills.
Taking these steps will set a solid foundation for your career transition from forensic biology to clinical trials.

2: Research clinical trials industry and roles
Understanding the landscape of clinical trials and various roles is crucial for a smooth career transition from forensic biology to clinical trials.
Actionable Steps:
- Dive into online research: Spend time exploring reputable websites and publications to grasp industry trends and updates in clinical research careers and pharmaceutical industry jobs.
- Identify key positions: Look for job descriptions to understand the responsibilities and qualifications required for different roles, including CRA (Clinical Research Associate) positions and regulatory affairs in clinical trials.
- Stay informed: Regularly read industry reports and follow relevant news to keep up with emerging developments in clinical trial management skills and patient recruitment strategies.
Explanation: These steps will give you a comprehensive view of the clinical trials field, helping you make informed career decisions when transitioning from forensic biology to clinical trials.
Staying updated on industry trends ensures that you remain relevant and competitive in the job market for clinical research careers.
For instance, regularly reading industry reports can provide insights into emerging technologies and methodologies that are shaping the future of clinical trials, such as advancements in clinical data management and site selection and feasibility in clinical studies.
This thorough research will prepare you for the next steps in your transition from forensic biology to clinical trials, including pursuing GCP (Good Clinical Practice) certification and learning about clinical trial protocol development.
3: Network with clinical research professionals
Building a strong professional network is crucial for successfully transitioning from forensic biology to clinical trials and advancing in clinical research careers.
Actionable Steps:
- Attend industry conferences and webinars: Participate in events to meet and interact with professionals in the clinical research field, including those in CRA (Clinical Research Associate) positions.
- Join online forums and LinkedIn groups: Engage in discussions and share insights to connect with industry experts and peers working in pharmaceutical industry jobs.
- Schedule informational interviews: Reach out to experienced clinical researchers to learn about their career paths, gain valuable advice on clinical trial management skills, and explore opportunities in regulatory affairs in clinical trials.
Explanation: These steps help you establish connections and gain insights into the clinical trials industry. Networking can open doors to job opportunities and provide mentorship in areas such as clinical data management and patient recruitment strategies.
For example, attending a conference can help you learn about the latest industry trends and innovations, including GCP (Good Clinical Practice) certification and clinical trial protocol development. Leveraging these relationships will significantly enhance your career transition strategy from forensic biology to clinical trials.
Networking is a powerful tool that can accelerate your journey into the clinical trials field, helping you explore various aspects like site selection and feasibility in clinical studies.

4: Gain relevant certifications (e.g., ACRP, SOCRA)
Earning certifications like ACRP and SOCRA is crucial for establishing credibility in the clinical trials field, especially when transitioning from forensic biology to clinical trials.
Actionable Steps:
- Research relevant certifications: Identify certifications that are highly regarded in the clinical trials industry, such as ACRP, SOCRA, and GCP (Good Clinical Practice) certification.
- Enroll in certification courses: Sign up for online or in-person courses that prepare you for certification exams and enhance your clinical trial management skills.
- Allocate study time: Dedicate specific hours each week to study and take practice exams to ensure you are well-prepared for clinical research careers.
Benefits of gaining certifications in clinical trials:
- Enhanced credibility in the field
- Improved job prospects and earning potential, including CRA (Clinical Research Associate) positions
- Expanded professional network in the pharmaceutical industry
Explanation: These steps are vital for demonstrating your commitment to the field and enhancing your skill set in clinical trial protocol development and regulatory affairs in clinical trials.
Certifications validate your expertise and make you more competitive in the job market for clinical research careers.
For example, the Association of Clinical Research Professionals (ACRP) offers certifications that are globally recognized, helping you stand out to potential employers in the pharmaceutical industry.
Investing time in gaining certifications will significantly accelerate your transition from forensic biology to clinical trials, paving the way for career growth in areas such as patient recruitment strategies and clinical data management.

5: Seek internships or entry-level positions
Securing internships or entry-level positions is crucial for gaining hands-on experience in the clinical trials field, especially when transitioning from forensic biology to clinical trials.
Actionable Steps:
- Apply for internships: Search job boards and company websites for internship opportunities in clinical trials and CRA (Clinical Research Associate) positions.
- Leverage networking connections: Use contacts from industry events and online forums to discover hidden job opportunities in clinical research careers and pharmaceutical industry jobs.
- Volunteer for clinical trials projects: Offer your time to gain practical exposure and build your resume, focusing on clinical trial management skills and patient recruitment strategies.
Explanation: These steps are vital for gaining practical experience and building your professional network. Internships and entry-level positions provide firsthand insights into the daily responsibilities and challenges of clinical trials, including clinical data management and regulatory affairs in clinical trials.
This exposure is invaluable for understanding the industry’s nuances and can significantly enhance your employability. For example, volunteering for projects can help you acquire skills that are highly sought after by employers, such as GCP (Good Clinical Practice) certification and clinical trial protocol development.
Gaining hands-on experience through these roles will pave the way for a successful career transition from forensic biology to clinical trials, enhancing your knowledge in areas like site selection and feasibility in clinical studies.

6: Tailor resume to highlight applicable experience
Tailoring your resume to highlight applicable experience is essential for standing out in the clinical trials job market when transitioning from forensic biology to clinical trials.
Actionable Steps:
- Use a targeted resume template: Choose a resume template that emphasizes your transferable skills and relevant experiences for clinical research careers.
- Include specific examples: Showcase examples of how your forensic biology skills apply to clinical trials roles, such as data analysis and clinical trial management skills.
- Highlight certifications and coursework: List any relevant certifications, training programs, or courses that enhance your qualifications for clinical trials, such as GCP (Good Clinical Practice) certification.
Key elements to include in your tailored resume for pharmaceutical industry jobs:
- Relevant technical skills and software proficiencies for clinical data management
- Specific achievements in data analysis or research applicable to CRA (Clinical Research Associate) positions
- Any experience with regulatory affairs in clinical trials or ethics
Explanation: These steps help you present a compelling case to potential employers by showcasing your relevant skills and experiences for clinical trial protocol development.
A well-tailored resume increases your chances of getting noticed in a competitive job market. For instance, including certifications can demonstrate your commitment and expertise in patient recruitment strategies and site selection and feasibility in clinical studies.
This approach aligns with industry standards, ensuring you are well-prepared for the transition from forensic biology to clinical trials.

Partner with Alleo on Your Career Transition Journey
We’ve explored the challenges of transitioning from forensic biology to clinical trials, and the steps to achieve it. But did you know you can work directly with Alleo to make this journey easier and faster?
Setting up an account with Alleo is simple. Start by signing up for a free 14-day trial—no credit card required.
Once you’re in, create a personalized career transition plan tailored to your unique skills and goals. Alleo’s AI coach will guide you through each step, offering specific advice on gaining certifications like GCP (Good Clinical Practice), networking for clinical research careers, and refining your resume for CRA (Clinical Research Associate) positions.
Alleo provides full coaching sessions, just like a human coach. The AI will follow up on your progress, adapt to any changes, and keep you accountable via text and push notifications.
This ensures you stay on track and motivated throughout your career transition from forensic biology to clinical trials.
Ready to get started for free? Let me show you how to launch your journey into pharmaceutical industry jobs and clinical trial management!
Step 1: Log In or Create Your Alleo Account
To begin your career transition journey, log in to your Alleo account or create a new one to access personalized AI coaching tailored to your move from forensic biology to clinical trials.

Step 2: Choose Your Career Transition Goal
Select “Setting and achieving personal or professional goals” to focus your efforts on transitioning from forensic biology to clinical trials. This goal aligns perfectly with your career shift, helping you create a structured plan to overcome challenges and acquire the necessary skills and experience for success in your new field.

Step 3: Select “Career” as Your Focus Area
Choose “Career” as your life area to focus on, as this aligns perfectly with your goal of transitioning from forensic biology to clinical trials, allowing Alleo’s AI coach to provide tailored guidance for your professional shift.

Step 4: Starting a coaching session
Begin your journey with an initial intake session to discuss your career transition goals and create a personalized action plan for moving from forensic biology to clinical trials.

Step 5: Viewing and Managing Goals After the Session
After your coaching session on transitioning from forensic biology to clinical trials, check the Alleo app’s home page to review and manage the career transition goals you discussed, ensuring you stay on track with your personalized action plan.
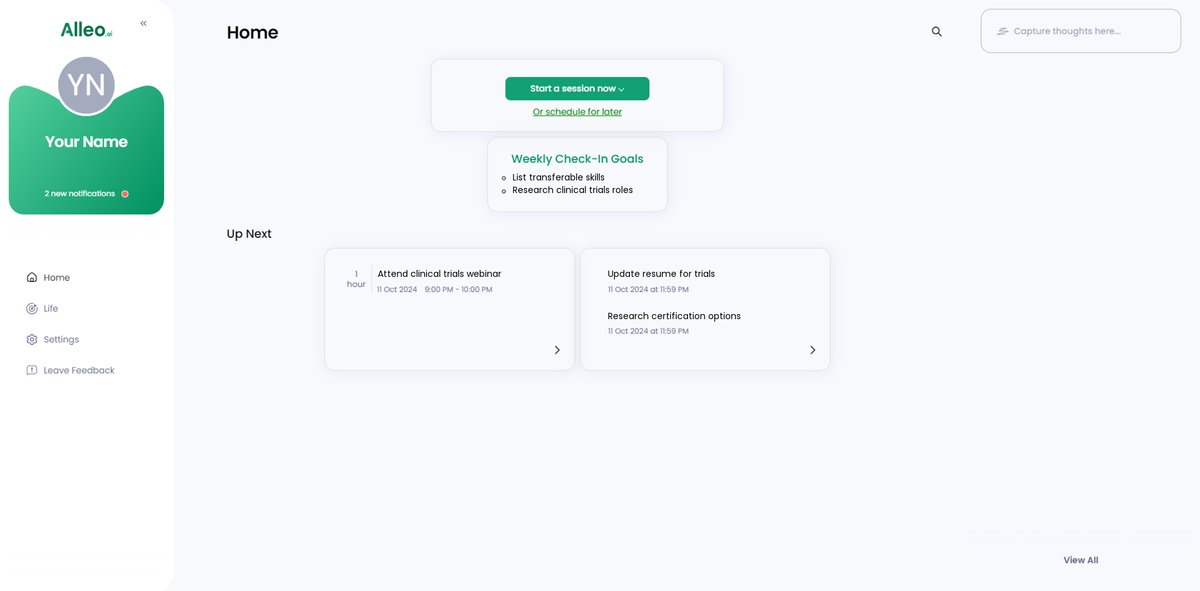
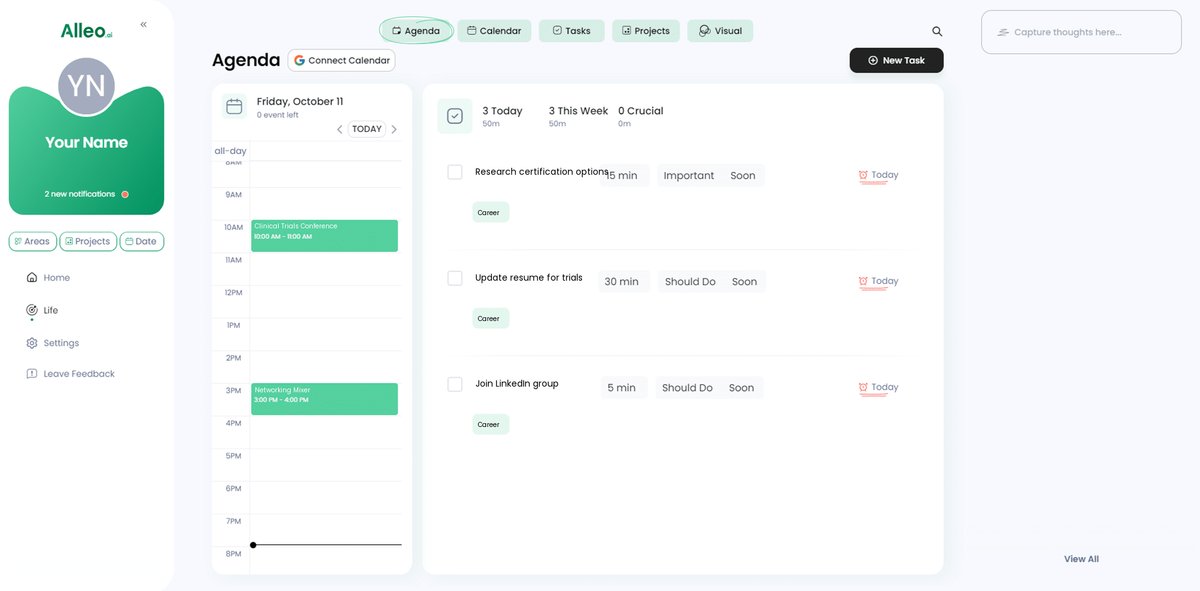
Step 6: Adding events to your calendar or app
Use Alleo’s calendar and task features to schedule and track key milestones in your career transition journey, such as certification exam dates, networking events, and application deadlines, ensuring you stay organized and motivated throughout the process.
Making Your Career Transition a Reality
You’ve taken in a wealth of information about transitioning from forensic biology to clinical trials. Now, it’s time to put these strategies into action.
Remember, while this shift from forensic biology to clinical trials may seem challenging, it’s entirely achievable with the right plan. Identify your transferable skills, research the pharmaceutical industry, network, and gain relevant experience in clinical research careers.
Don’t forget to tailor your resume to highlight your applicable experience, including any clinical trial management skills you’ve developed.
Empower yourself with certifications like GCP (Good Clinical Practice) and hands-on experience in areas such as patient recruitment strategies or clinical data management.
Most importantly, stay persistent and proactive in your journey towards CRA (Clinical Research Associate) positions or other roles in clinical trials.
For personalized support, consider leveraging Alleo’s AI life coach. Try Alleo for free to guide you through your career transition from forensic biology to clinical trials.
You’ve got this!